In an industry where safety, precision, and compliance drive innovation, Meridian LMS ensures your teams stay trained, certified, and ready. Simplify learning management while maintaining the highest regulatory and quality standards.

In the life sciences industry—where innovation meets strict regulation—effective learning management is critical. Whether you’re in pharmaceuticals, biotechnology, or medical device manufacturing, ensuring your workforce is trained, certified, and compliant is essential to bringing safe, effective products to market.
Meridian LMS helps life sciences organizations simplify compliance management, accelerate onboarding, and support continuous learning across global teams. Our secure, scalable learning platform empowers employees, partners, and contractors to maintain readiness, reduce risk, and meet FDA, EMA, and ISO requirements with confidence.
Track and verify completion of GMP, GCP, GLP, and other required programs. Meridian automates documentation and provides robust audit trails that support FDA 21 CFR Part 11, EMA, and ISO requirements.

Deliver structured, role-based learning paths that help new hires and lab personnel reach competency faster. Automated certification tracking, renewals, and alerts keep your teams continuously compliant.
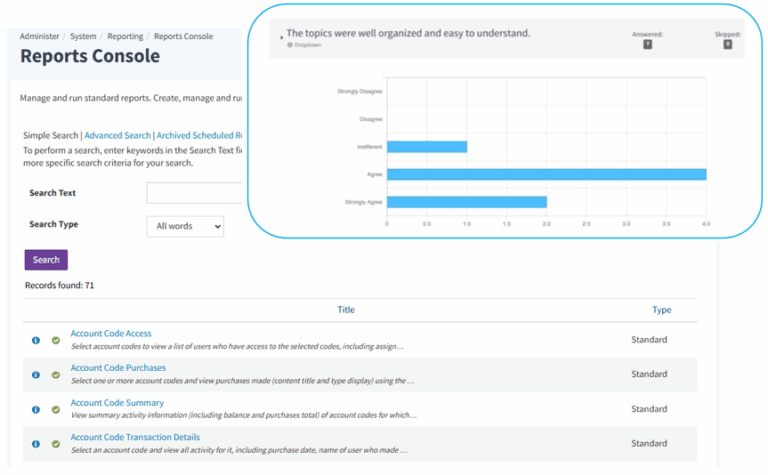
Track performance with dashboards and detailed reports; utilize scheduled data exports for informed decision-making.
Train employees, partners, and third-party vendors across multiple regions and languages with centralized oversight. Maintain data integrity while providing consistent learning experiences for distributed teams.
Reinforce SOPs, product quality standards, and safety protocols through blended learning and mobile-ready microlearning—ensuring teams remain aligned as requirements evolve.

Combine online, in-person, and hybrid learning formats to provide flexible experiences. Meridian LMS supports blended sessions where participants can engage in the same event—some attending in person with an instructor and others joining online—enhancing flexibility and inclusivity.
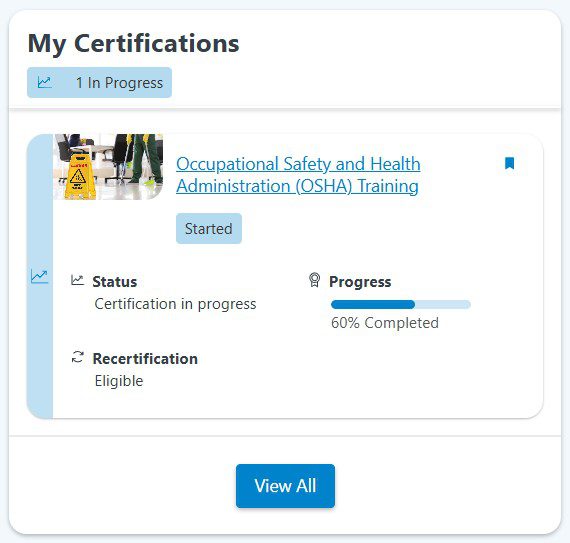
Identify skill gaps and ensure employees meet scientific, technical, and operational competencies.
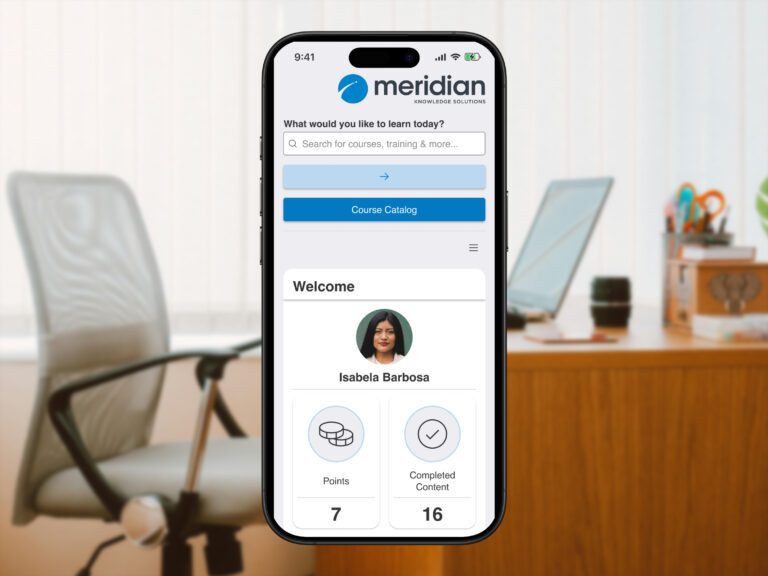
Allow team members to access learning materials anytime, anywhere, increasing convenience and completion rates.

Connect with HRIS, LIMS, QMS, Salesforce, Microsoft 365, and other critical systems for streamlined workflows.

Manage multiple facilities, divisions, or partners within one secure, centralized LMS environment.
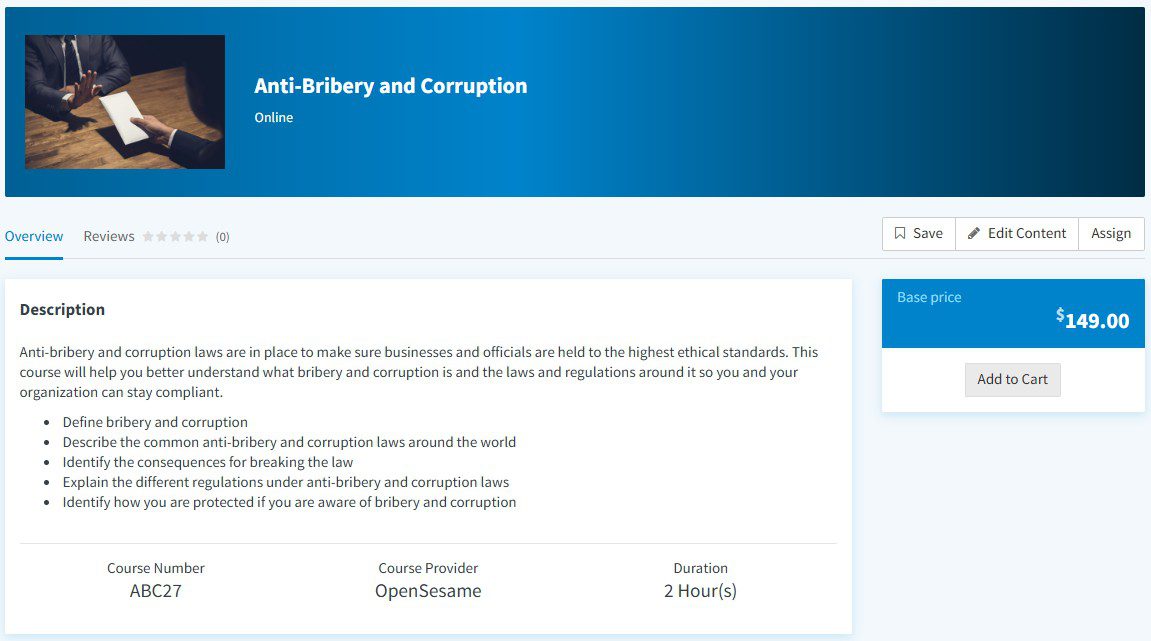
Monetize your training and certification programs by offering paid courses and securely managing member payments through the LMS.
Meridian LMS helps life sciences organizations not only meet regulatory requirements—but exceed them. By unifying training, reporting, and workforce analytics, you can ensure your teams are always prepared, aligned, and ready to innovate safely and efficiently.
Yes. Meridian LMS integrates with leading HRIS, LIMS, and QMS platforms, as well as Salesforce, Microsoft 365, and Oracle—ensuring seamless data flow across your ecosystem.
Absolutely. With FedRAMP-authorized cloud hosting and private or on-premises deployment options, Meridian meets the strictest data protection and compliance standards.
Certification paths, expiration alerts, and renewals are automated to keep every employee up to date with regulatory requirements.
Yes. Meridian supports multilingual training delivery for consistent learning experiences across global regions and time zones.